Artificial Intelligence (AI) is revolutionizing the medical device industry, offering innovative solutions for risk assessment and management. In a highly regulated sector, accurate and efficient risk assessment is critical to ensuring patient safety, meeting compliance requirements, and accelerating product development. AI-driven tools provide unparalleled capabilities to analyze, predict, and mitigate risks throughout the lifecycle of a medical device.
This blog explores the transformative role of AI in medical device risk assessment, its benefits, applications, and key considerations for leveraging this technology effectively.
Why Risk Assessment is Crucial in Medical Devices
Medical device risk assessment involves identifying, analyzing, and mitigating potential hazards associated with a device. It ensures compliance with standards like ISO 14971 (risk management for medical devices) and addresses critical concerns such as:
- Patient safety
- Regulatory approval
- Product reliability
- Market confidence
Traditionally, risk assessment has been a manual, time-intensive process. AI now offers a paradigm shift by automating and enhancing these processes, making them faster, more accurate, and scalable.
How AI Enhances Medical Device Risk Assessment
AI-powered technologies bring efficiency, accuracy, and predictive capabilities to risk assessment processes. Here’s how:
1. Automated Hazard Identification
- AI tools can analyze vast datasets, including historical data, clinical studies, and adverse event reports, to identify potential hazards.
- Machine learning algorithms detect patterns that might be overlooked by human analysis.
2. Predictive Risk Modeling
- AI uses predictive analytics to foresee potential device failures or adverse outcomes.
- This helps manufacturers proactively address risks before devices reach the market.
3. Real-Time Monitoring
- AI-powered systems monitor devices post-market, identifying emerging risks through real-time data from users, healthcare providers, and connected devices.
4. Risk-Benefit Analysis
- AI tools assess the balance between risks and benefits more comprehensively, supporting data-driven decision-making during device design and development.
5. Streamlined Documentation
- AI automates the creation of risk management files and documentation, ensuring compliance with regulatory standards like ISO 14971 and FDA requirements.
Key Benefits of AI in Medical Device Risk Assessment
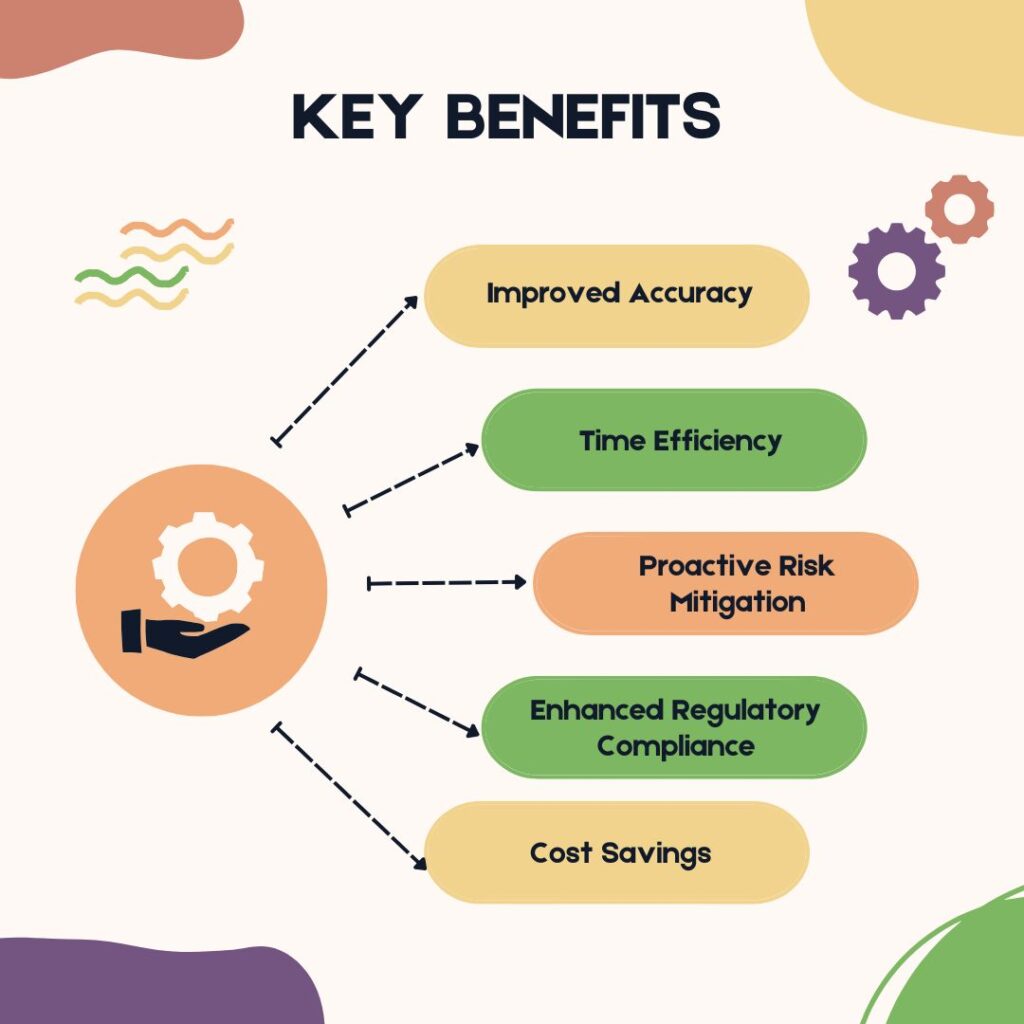
- Improved Accuracy
- AI minimizes human errors by providing consistent and precise risk evaluations.
- AI minimizes human errors by providing consistent and precise risk evaluations.
- Time Efficiency
- Automated processes significantly reduce the time required for risk analysis and documentation.
- Automated processes significantly reduce the time required for risk analysis and documentation.
- Proactive Risk Mitigation
- Predictive models help address potential risks early, reducing post-market recalls and adverse events.
- Predictive models help address potential risks early, reducing post-market recalls and adverse events.
- Enhanced Regulatory Compliance
- AI ensures adherence to global standards by integrating compliance checkpoints within workflows.
- AI ensures adherence to global standards by integrating compliance checkpoints within workflows.
- Cost Savings
- Early risk detection and mitigation reduce costly redesigns and regulatory setbacks.
- Early risk detection and mitigation reduce costly redesigns and regulatory setbacks.
Applications of AI in Risk Assessment
1. Design and Development
- AI tools evaluate risks during the design phase, optimizing device features to minimize hazards.
2. Clinical Trials
- Machine learning algorithms predict potential risks associated with device usage during trials, improving trial safety and efficiency.
3. Post-Market Surveillance
- AI analyzes real-world data to identify emerging risks and ensures continuous monitoring of devices after market launch.
4. Regulatory Submissions
- AI automates risk assessment documentation, simplifying regulatory submissions and reducing errors.
5. Cybersecurity Risk Management
- For connected devices, AI assesses cybersecurity risks, protecting patient data and device functionality.
Challenges and Considerations

- Data Quality and Availability
- AI systems rely on high-quality, comprehensive datasets. Incomplete or biased data can lead to inaccurate assessments.
- AI systems rely on high-quality, comprehensive datasets. Incomplete or biased data can lead to inaccurate assessments.
- Regulatory Acceptance
- While AI is transforming risk assessment, regulatory bodies may require clear validation of AI-generated results.
- While AI is transforming risk assessment, regulatory bodies may require clear validation of AI-generated results.
- Interpretability of AI Models
- Complex AI algorithms must provide explainable results for manufacturers and regulators to trust the analysis.
- Complex AI algorithms must provide explainable results for manufacturers and regulators to trust the analysis.
- Integration with Existing Systems
- AI tools should integrate seamlessly into existing workflows without disrupting processes.
- AI tools should integrate seamlessly into existing workflows without disrupting processes.
How Bioexcel Supports AI-Driven Risk Assessment
At Bioexcel, we help medical device manufacturers leverage AI to enhance their risk assessment and compliance efforts. Our services include:
- AI-Powered Risk Modeling: Advanced tools for predictive risk analysis and mitigation.
- Regulatory Compliance Support: Ensuring adherence to global standards like ISO 14971 and FDA guidelines.
- Post-Market Surveillance Solutions: AI systems for real-time monitoring and risk management.
- Custom AI Integration: Tailored AI solutions that fit seamlessly into your existing workflows.
With Bioexcel’s expertise, you can harness AI to streamline risk assessment, improve safety, and achieve regulatory success.
Conclusion: Transforming Risk Assessment with AI
The integration of AI into medical device risk assessment marks a new era of efficiency, precision, and innovation. By adopting AI-driven tools, manufacturers can ensure safer devices, faster compliance, and reduced costs—all while gaining a competitive edge in a rapidly evolving industry.
Ready to leverage AI for your medical device risk assessment? Contact Bioexcel today to explore tailored solutions that meet your needs.