- Our Services
Biostatistics
- Accurate Statistical Analysis for Medical Device and Pharmaceutical Success
Biostatistics Services: Turning Data into Actionable Insights
Biostatistics
Unveiling Data Insights for Better Healthcare
At Bioexcel, we offer comprehensive biostatistics services to ensure the accuracy, reliability, and regulatory compliance of your clinical data. Our expertise in medical device and pharmaceutical biostatistics enables evidence-based decision-making, helping you meet regulatory requirements and achieve successful trial outcomes.
Why Biostatistics Matters:
Biostatistics is a critical component of clinical trials, ensuring that data is analyzed and interpreted accurately. Proper statistical analysis validates the safety, efficacy, and performance of medical devices and pharmaceuticals, supporting regulatory submissions and market approvals.
Key Benefits:
- Regulatory Compliance: Meet global standards like ISO 14155, FDA, and MDR.
- Data Integrity: Ensure accuracy and reliability in clinical trial results.
- Informed Decision-Making: Gain actionable insights from complex datasets.

Our Biostatistics Services

Study Design & Sample Size Calculation
Optimized Study Designs for Accurate Results
We provide guidance on study design, including sample size calculations and statistical methodology, to ensure your trial is scientifically robust and resource-efficient.
Key Features:
- Sample size determination based on power analysis.
- Adaptive and Bayesian trial designs for flexibility.
- Endpoints selection for safety and performance evaluation.
Statistical Analysis Plans (SAP)
Blueprint for Data Analysis
We develop comprehensive Statistical Analysis Plans (SAPs) tailored to your study objectives and regulatory requirements.
Key Features:
- Definition of statistical methods and models.
- Pre-specified data handling and imputation strategies.
- Alignment with regulatory guidelines and trial objectives.
Data Analysis and Reporting
Transforming Data into Evidence
Our biostatisticians provide detailed data analysis and generate regulatory-compliant reports that support your trial outcomes.
Key Features:
- Interim and final data analysis.
- Preparation of Clinical Study Reports (CSR) and appendices.
- Data visualization for easy interpretation of results.
Real-World Evidence (RWE) Analysis
Harnessing Real-World Data for Strategic Insights
We analyze real-world data to demonstrate long-term safety and performance of medical devices, supporting regulatory submissions and post-market surveillance.
Key Features:
- RWE collection and integration into clinical evidence.
- Advanced statistical modeling for trend analysis.
- Insights for device improvements and compliance.
Interim Analysis and DMC Support
Real-Time Monitoring for Better Decision-Making
We provide interim analysis to support Data Monitoring Committees (DMCs), ensuring timely and informed decisions during your trial.
Key Features:
- Early assessment of trial safety and efficacy.
- Recommendations for trial modifications if needed.
- Comprehensive support for DMC reporting and meetings.
Post-Market Data Analysis
Ongoing Safety and Performance Validation
We analyze post-market data to ensure continuous compliance and improve device performance in real-world use.
Key Features:
- PMS and PMCF data analysis.
- Preparation of Periodic Safety Update Reports (PSUR).
- Statistical review for risk management updates.
Case Studies
Case Study 1: Sample Size Optimization for an Orthopedic Implant Trial
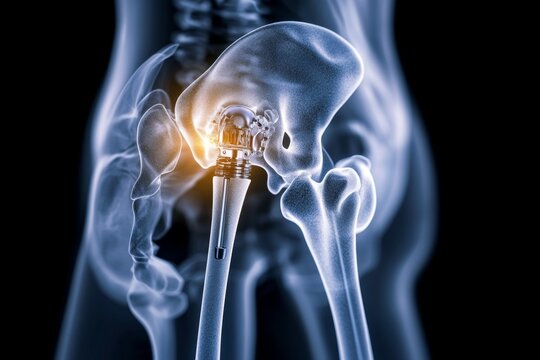
Challenge:
A client developing a Class III orthopedic implant faced challenges in determining the optimal sample size for their clinical trial while meeting regulatory requirements.
Solution by Bioexcel:
- Conducted power analysis to determine the minimum sample size needed to detect clinically significant differences.
- Designed an adaptive trial to account for potential variability in patient responses.
- Developed a Statistical Analysis Plan (SAP) aligned with MDR requirements.
Outcome:
- Reduced trial costs by 15% through optimized sample size.
- Achieved regulatory approval for the trial with a robust statistical framework.
Challenge:
An intraocular lens (IOL) manufacturer needed statistical support for analyzing clinical data on visual acuity improvement in patients.
Solution by Bioexcel:
- Conducted interim and final analysis of clinical endpoints, including visual acuity, patient-reported outcomes, and adverse events.
- Developed data visualization tools to present results clearly to regulatory authorities.
- Prepared Clinical Study Reports (CSR) for CE marking under MDR.
Outcome:
- Demonstrated a 25% improvement in visual outcomes compared to competitors.
- Supported the client’s successful CE marking application.
Case Study 2: Statistical Analysis for an Ophthalmology Device

Case Study 3: RWE Analysis for a Hemostatic Device

Challenge:
A hemostatic device manufacturer needed to analyze real-world data to validate long-term safety and performance for their PMCF study.
Solution by Bioexcel:
- Collected and analyzed data from surgical registries and post-market feedback.
- Used advanced statistical models to assess trends in bleeding time reduction and adverse events.
- Prepared a comprehensive PMCF report for regulatory submission.
Outcome:
- Demonstrated consistent performance with a 40% reduction in bleeding time.
- Enabled successful compliance with EU MDR post-market requirements.
Why Choose Bioexcel for Biostatistics?
Expert Biostatisticians
A team of experienced professionals skilled in advanced statistical methodologies and global regulatory standards.
Regulatory Alignment
Deep understanding of ISO, FDA, MDR, and ICH guidelines ensures compliance at every step.
Customized Solutions
Tailored biostatistics support for all device classes, including Class III, IVDs, and combination products.
Proven Track Record
Demonstrated success across diverse therapeutic areas, including orthopedics, ophthalmology, and hemostatic devices.

Help & FAQ
Frequently Asked Questions
Unlike drug trials, medical device trials require performance-based endpoints, usability testing, and real-world evidence analysis. Our biostatistics services address these unique needs while ensuring regulatory compliance.
Yes, we specialize in adaptive and Bayesian trial designs, allowing flexibility and resource optimization during trials.
An SAP includes detailed statistical methodologies, data handling procedures, endpoint definitions, and analysis models aligned with regulatory guidelines.
Transform your clinical data into actionable insights with Bioexcel’s expert biostatistics services. Contact us today to ensure regulatory compliance and successful trial outcomes for your medical device or pharmaceutical product.








