Post-Market Clinical Follow-Up (PMCF) Plan for Medical Devices
Strengthen Your Clinical Evidence with a Compliant PMCF Strategy
Under the EU Medical Device Regulation (EU MDR 2017/745), Post-Market Clinical Follow-Up (PMCF) is no longer optional—it’s a mandatory and strategic activity that ensures the ongoing safety, performance, and clinical benefit of your device throughout its lifecycle.
At Bioexcel, we design, implement, and manage MDR-compliant PMCF Plans and PMCF Evaluation Reports (PERs) that seamlessly integrate with your Clinical Evaluation Report (CER), Post-Market Surveillance (PMS) system, and Risk Management File (RMF).
What is a PMCF Plan and Why It Matters
A Post-Market Clinical Follow-Up Plan is a structured roadmap that defines how a manufacturer will proactively collect and evaluate clinical data after CE marking to confirm device safety and performance under real-world conditions.
PMCF is an essential part of the continuous clinical evaluation process and supports regulatory expectations under:
-
Annex XIV, Part B of EU MDR 2017/745
-
MDCG 2020-7 guidance on PMCF Plan and Evaluation Report
-
ISO 14155:2020 (for PMCF clinical investigations)
-
MEDDEV 2.12/2 rev 2 and IMDRF PMS frameworks
A robust PMCF plan demonstrates regulatory maturity and ensures that your device remains safe, effective, and compliant—especially when technology, user practice, or clinical environments evolve.

How PMCF Integrates Into the Device Lifecycle
The PMCF process is not standalone. It is interconnected with your other key regulatory documents:
| Document | PMCF Link |
|---|---|
| Clinical Evaluation Report (CER) | PMCF data closes evidence gaps and supports ongoing clinical evaluation. |
| Risk Management File (RMF) | PMCF findings validate or update identified risks. |
| Post-Market Surveillance (PMS) Plan | PMCF is part of your proactive PMS activities. |
| Periodic Safety Update Report (PSUR) | PMCF results feed into benefit–risk reassessment. |
| Summary of Safety & Clinical Performance (SSCP) | PMCF outcomes enhance transparency to users and patients. |
At Bioexcel, we ensure that all these components form a cohesive, audit-ready ecosystem for your notified body submission.
Proactive and Reactive PMCF Activities
A compliant PMCF plan must balance proactive and reactive data collection strategies.
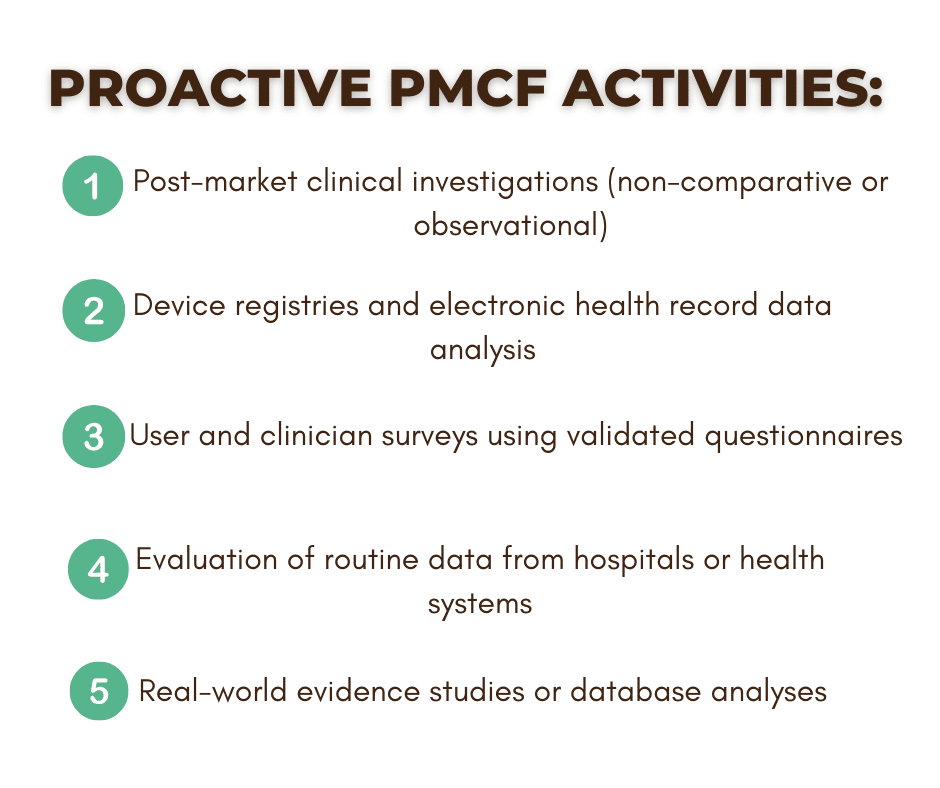
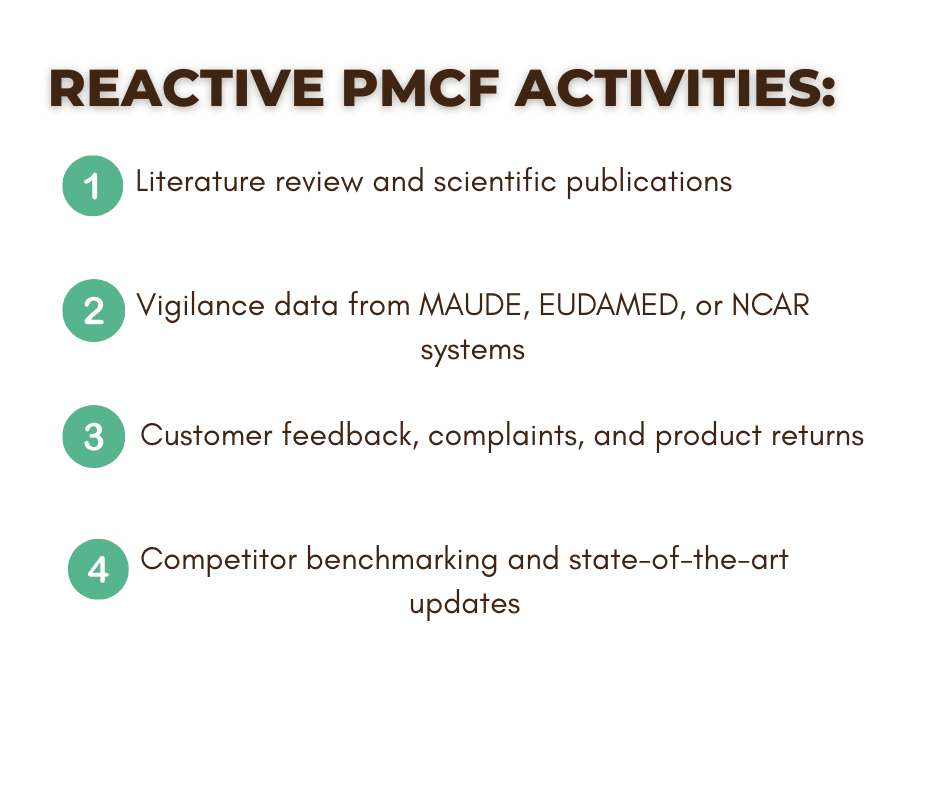
What Bioexcel Offers

PMCF Plan Development

PMCF Study Design and Execution

Surveys and Registry Data

PMCF Evaluation Report (PER) Preparation
Industries & Devices We Support
Bioexcel provides PMCF support for:
Orthopedic and spine implants
Cardiovascular and catheter-based devices
Surgical consumables and wound care
SaMD and AI-based diagnostic software
In-vitro diagnostic (IVD) devices (PMCF equivalents under IVDR)
Start Your PMCF Journey with Bioexcel
Whether you need a new PMCF Plan or want to update your existing documentation for MDR compliance, Bioexcel can help you build a structured, evidence-based, and regulator-approved system.








