In the evolving healthcare landscape, Software as a Medical Device (SaMD) is revolutionizing patient care, diagnostics, and treatment. With the rise of AI-powered medical device innovations, SaMD is reshaping how healthcare providers and patients interact with technology. For companies in the medical device industry, understanding SaMD’s potential and regulatory requirements is critical for staying ahead.
In this blog, we’ll explore what SaMD is, its applications in healthcare, key benefits, and how AI is driving innovation.
What is Software as a Medical Device (SaMD)?
Software as a Medical Device (SaMD) is defined by the International Medical Device Regulators Forum (IMDRF) as:
“Software intended to be used for medical purposes without being part of a hardware medical device.”
Unlike traditional devices, SaMD is purely software-based and can perform functions like:
- Diagnosis of diseases
- Monitoring and managing medical conditions
- Supporting clinical decision-making
Examples of SaMD:
- AI algorithms that analyze medical imaging (e.g., X-rays, CT scans)
- Mobile apps that monitor vital signs like heart rate
- Software platforms predicting disease progression through data analytics
The Role of AI in SaMD Innovations
Artificial Intelligence (AI) is a game-changer in the development and functionality of SaMD. AI-powered SaMD uses machine learning, deep learning, and advanced algorithms to deliver faster, more accurate, and data-driven healthcare solutions.
Key Applications of AI in SaMD:
- Medical Imaging and Diagnostics:
- AI algorithms analyze medical images to detect early signs of diseases like cancer, fractures, or neurological disorders.
- Example: AI-powered software for tumor detection in mammograms.
- AI algorithms analyze medical images to detect early signs of diseases like cancer, fractures, or neurological disorders.
- Remote Patient Monitoring (RPM):
- SaMD enables real-time monitoring of patients’ vital data, helping doctors provide personalized care.
- Example: AI tools that monitor heart rhythms to detect arrhythmias.
- SaMD enables real-time monitoring of patients’ vital data, helping doctors provide personalized care.
- Predictive Analytics:
- AI-driven SaMD predicts disease progression or potential risks by analyzing large datasets.
- Example: Diabetes prediction based on historical glucose trends.
- AI-driven SaMD predicts disease progression or potential risks by analyzing large datasets.
- Clinical Decision Support Systems (CDSS):
- AI helps healthcare providers make informed decisions based on real-time data analysis.
- Example: Software assisting in treatment plans for complex medical conditions.
- AI helps healthcare providers make informed decisions based on real-time data analysis.
- Digital Therapeutics:
- Software solutions that provide evidence-based therapeutic interventions for managing mental health, diabetes, and other conditions.
- Example: Cognitive Behavioral Therapy (CBT)-based mobile apps.
- Software solutions that provide evidence-based therapeutic interventions for managing mental health, diabetes, and other conditions.
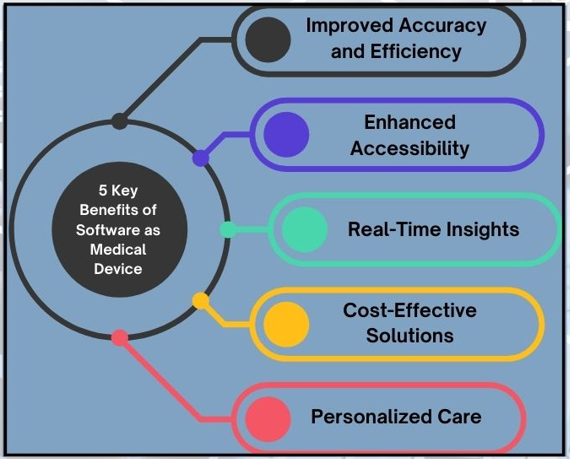
Key Benefits of AI-Powered SaMD in Healthcare
- Improved Accuracy and Efficiency:
- AI reduces human error and increases diagnostic precision, saving time and resources.
- AI reduces human error and increases diagnostic precision, saving time and resources.
- Enhanced Accessibility:
- SaMD enables remote care, benefiting patients in rural or underserved areas.
- SaMD enables remote care, benefiting patients in rural or underserved areas.
- Real-Time Insights:
- Continuous data monitoring allows healthcare providers to make faster, data-driven decisions.
- Continuous data monitoring allows healthcare providers to make faster, data-driven decisions.
- Cost-Effective Solutions:
- Reduces the need for frequent in-person consultations and hospital visits.
- Reduces the need for frequent in-person consultations and hospital visits.
- Personalized Care:
- AI-powered tools deliver tailored treatment plans based on patient-specific data.
- AI-powered tools deliver tailored treatment plans based on patient-specific data.
Regulatory Requirements for SaMD
Given its potential to impact patient outcomes, SaMD is subject to strict regulatory oversight.
1. International Standards:
- IMDRF Guidelines: Establishes the global framework for SaMD classification and risk-based evaluation.
- ISO 13485: Quality Management System (QMS) for SaMD development and lifecycle management.
2. Regional Regulations:
- USFDA: Under FDA’s Digital Health Center of Excellence, SaMD is classified based on risk.
- EU MDR: SaMD falls under EU MDR 2017/745, requiring CE marking and Clinical Evaluation Reports (CER).
- CDSCO (India): Classifies and approves SaMD under the Medical Device Rules, 2017.
3. Risk-Based Classification:
SaMD is classified based on its impact on patient outcomes:
- Class I: Low risk
- Class II: Moderate risk
- Class III: High risk
Challenges in SaMD Adoption
- Regulatory Complexity:
Navigating evolving global regulations can be challenging for manufacturers. - Data Privacy and Security:
Protecting patient data while maintaining compliance with regulations like HIPAA and GDPR. - Integration with Existing Systems:
Ensuring compatibility with hospital systems and electronic health records (EHRs). - Clinical Validation:
Demonstrating safety, accuracy, and performance through clinical trials. - AI Bias and Interpretability:
Reducing bias in AI algorithms to ensure equitable healthcare outcomes.

How Bioexcel Helps Navigate SaMD Innovation and Compliance
At Bioexcel, we provide expert solutions to help medical device companies bring AI-powered SaMD to market efficiently and compliantly. Our services include:
- Regulatory Strategy Development:
- Navigating regional and international regulatory pathways for SaMD approval.
- Navigating regional and international regulatory pathways for SaMD approval.
- Clinical Evaluation and Validation:
- Assisting with Clinical Evaluation Reports (CER) and clinical trial planning.
- Assisting with Clinical Evaluation Reports (CER) and clinical trial planning.
- QMS Implementation:
- Developing ISO 13485-compliant Quality Management Systems for SaMD lifecycle management.
- Developing ISO 13485-compliant Quality Management Systems for SaMD lifecycle management.
- Documentation Support:
- Preparing risk management reports, technical files, and post-market surveillance plans.
- Preparing risk management reports, technical files, and post-market surveillance plans.
- AI Algorithm Validation:
- Supporting clinical validation and ensuring transparency in AI-powered tools.
- Supporting clinical validation and ensuring transparency in AI-powered tools.
With Bioexcel’s guidance, you can successfully develop, validate, and launch SaMD while meeting regulatory requirements.
Conclusion: The Future of AI-Powered SaMD
Software as a Medical Device (SaMD) powered by AI is transforming healthcare delivery by improving accuracy, accessibility, and efficiency. While challenges remain, the benefits of AI-driven SaMD are undeniable. By leveraging innovative solutions and ensuring regulatory compliance, companies can unlock the full potential of SaMD to improve patient outcomes.
Ready to bring your AI-powered SaMD to market? Contact Bioexcel today for expert guidance on innovation, validation, and compliance.












