
The Central Drugs Standard Control Organization (CDSCO) governs the registration of medical devices in India. As the medical device industry grows, understanding the CDSCO process becomes essential. This blog provides a concise overview of the CDSCO registration process, its importance, and key steps involved.
Understanding CDSCO and Its Role
CDSCO, under the Ministry of Health and Family Welfare, acts as India’s national regulatory authority. It ensures the safety, efficacy, and quality of drugs and medical devices. The organization enforces regulations under the Drugs and Cosmetics Act, 1940, and the Medical Devices Rules, 2017, which classify devices based on risk levels.
Importance of CDSCO Registration
Manufacturers and importers must register their devices with CDSCO before marketing them in India. This registration serves as a legal requirement that ensures the device meets safety and performance standards. By doing so, manufacturers protect public health and enhance the credibility of their products in the market.
Classification of Medical Devices
A key initial step involves understanding the classification of your device. CDSCO categorizes devices into four classes based on risk:
- Class A: Low-risk devices (e.g., surgical gloves)
- Class B: Low-to-moderate risk devices (e.g., hypodermic needles)
- Class C: Moderate-to-high risk devices (e.g., bone fixation plates)
- Class D: High-risk devices (e.g., heart valves)
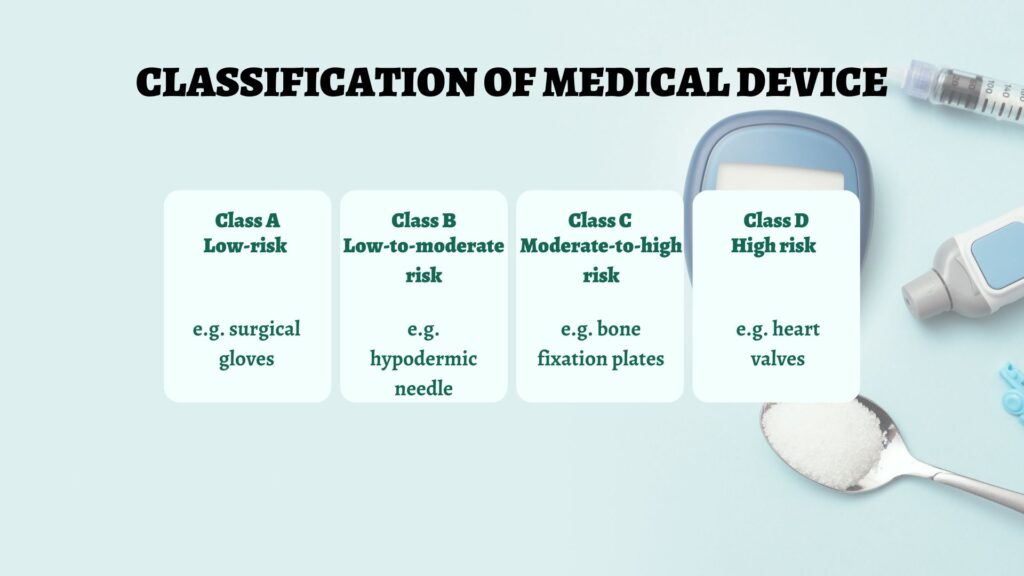
Each class requires specific regulatory measures, with higher-risk devices undergoing more rigorous scrutiny.
Steps in the CDSCO Registration Process
- Determine the Regulatory Pathway
- The classification of your device dictates whether you need an Import License (for foreign manufacturers) or a Manufacturing License (for domestic manufacturers). State authorities handle Class A and B devices, while CDSCO oversees Class C and D devices.
- Appoint an Authorized Indian Agent
- Foreign manufacturers need to appoint an Authorized Indian Agent. This agent acts as the point of contact with CDSCO and manages the registration process.
- Prepare the Application
- Gather all necessary documents, such as:
- A covering letter
- Duly filled application forms (Form MD-14 for import, Form MD-3 for manufacturing)
- Device Master File (DMF) and Plant Master File (PMF)
- Proof of fee payment
- An undertaking regarding the accuracy of the information
- Gather all necessary documents, such as:
- Submit the Application
- Submit the application online through the SUGAM portal. Ensure that you upload all documents correctly and pay the required fees.
- Technical Review and Evaluation
- CDSCO reviews the documents to assess the device’s safety, efficacy, and quality. For Class C and D devices, you might need to provide additional data, such as clinical trial reports.
- Inspection and Audit
- For domestic manufacturers, CDSCO may inspect the manufacturing facility to ensure compliance with Good Manufacturing Practices (GMP). Similarly, they might audit the manufacturing sites of imported devices.
- Grant of Registration
- Once CDSCO approves the application, they issue a registration certificate. This certificate allows the device to be legally marketed in India, and it typically lasts for five years, after which you must renew it.

Post-Registration Compliance
Compliance doesn’t end with registration. Manufacturers or importers must follow ongoing requirements, such as:
- Reporting adverse events
- Ensuring consistent quality control
- Submitting periodic safety updates
- Renewing the registration before it expires
Challenges and Best Practices
Foreign manufacturers often find the CDSCO registration process complex, especially if they are unfamiliar with Indian regulations. Common challenges include understanding regulatory requirements and maintaining compliance.
Best Practices:
- Engage Regulatory Experts: Consulting with regulatory experts can streamline the process and reduce errors.
- Stay Updated with Regulations: Regularly check for updates in Indian medical device regulations.
- Thorough Documentation: Ensure that you complete, accurately prepare, and organize all documentation.
Conclusion
Successfully navigating the CDSCO registration process is critical for bringing medical devices to the Indian market. By understanding device classification, preparing thorough documentation, and adhering to regulatory requirements, manufacturers and importers can achieve successful registration. This blog aims to demystify the process, providing valuable insights for those entering the Indian medical device market. Whether you are a domestic or international player, following these steps will help ensure successful registration and compliance.




