
CDSCO regulates medical devices in India. Companies must obtain a manufacturing license from CDSCO to legally manufacture devices and ensure regulatory compliance.
What is the CDSCO Manufacturing License?
The CDSCO Manufacturing License falls under the CLAA (Central Licensing Authority Approval) Scheme, granted by the State Licensing Authority and approved by the CLAA, provided the licensee adheres to all Medical Device Rules.
Many companies in India import raw materials, semi-finished products, or components. To complete the final assembly and packaging of medical devices within India, a CDSCO manufacturing license is mandatory. If the product is fully finished outside India, a CDSCO import license is needed instead.
How to Apply for a CDSCO Manufacturing License?
To register for a CDSCO manufacturing license in India, follow these steps:
- Identify License Type: Determine the specific license required for your device based on its classification.
- Facility Compliance: Ensure your manufacturing facility meets Good Manufacturing Practices (GMP) and eligibility standards.
- Qualified Personnel: Employ qualified technical staff to manage production processes.
- Submit Application: Complete the necessary forms, including Form 27, and provide documents like the Site Master File, premises details, manufacturing processes, and applicable fees to the State Drug Control Department or CDSCO.
Document Requirements for CDSCO Registration
To begin the registration process, the following documents are necessary:
- Address proof (e.g., certificate of registration, certificate of incorporation, Import-export certificate, or corporate site telephone bills).
- ID proof of an authorized individual responsible for handling registration processes.
Phases of the CDSCO Manufacturing License Process
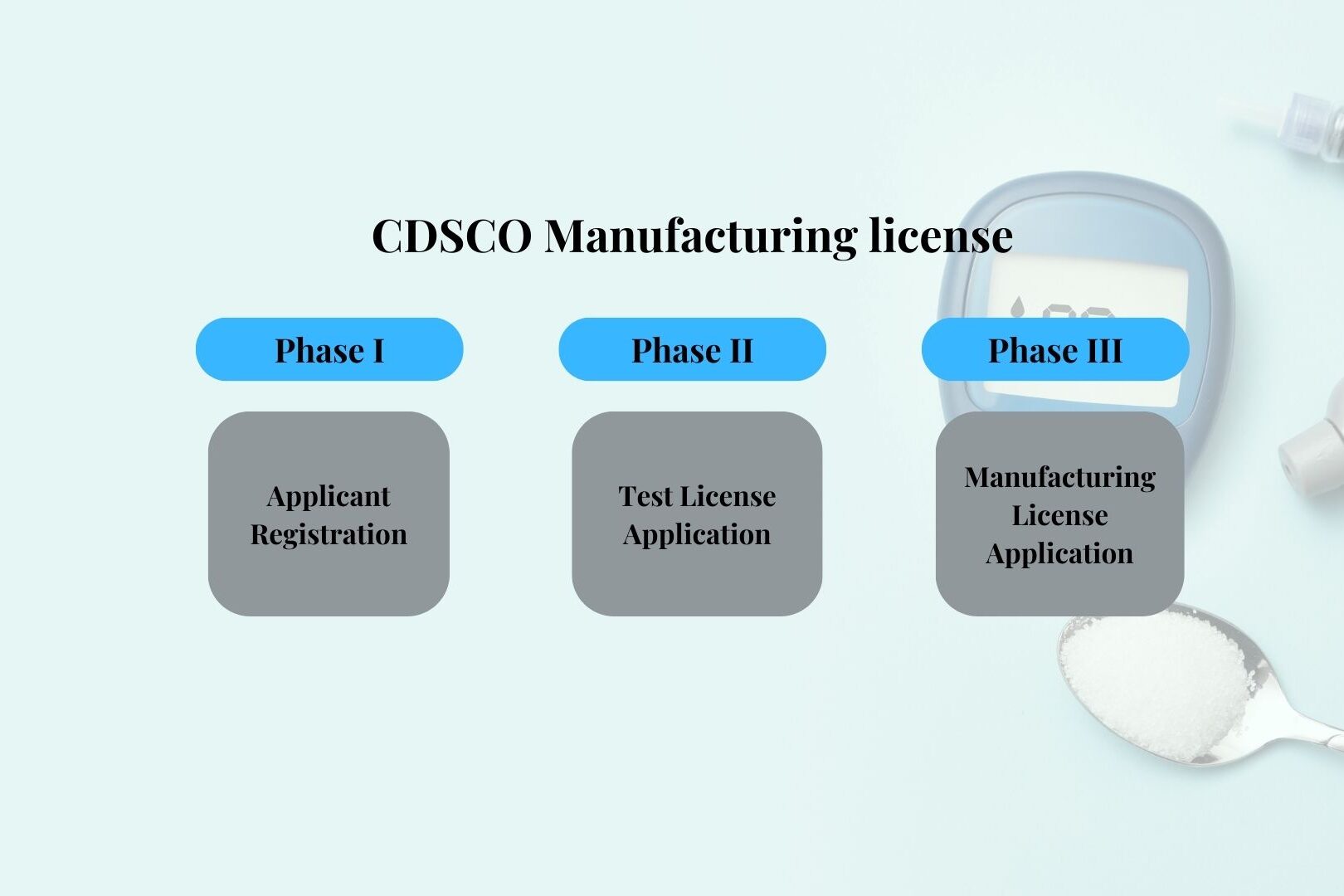
Phase I – Applicant Registration
In this phase, you must create a CDSCO portal login by uploading the required documents. The process involves:
- Submitting the CDSCO online form.
- Uploading company details.
- Receiving preliminary approval via email, followed by instructions for submitting hard copies.
Phase II – CDSCO Manufacturing Test License Application
A Test License (Form MD-12) is needed to manufacture small quantities of medical devices for testing, evaluation, and personnel training. The steps include:
- Online application submission.
- Document upload.
- Fee payment.
- Monitoring application status.
Phase III – CDSCO Manufacturing License Application
For commercial manufacturing of medical devices, follow these steps:
- Submit the online application through the CDSCO portal.
- For Class A & B devices, fill out Form MD-3; for Class C & D devices, fill Form MD-7.
- Upload all required documents.
- Pay the applicable fees.
- Receive an approval or rejection notification.
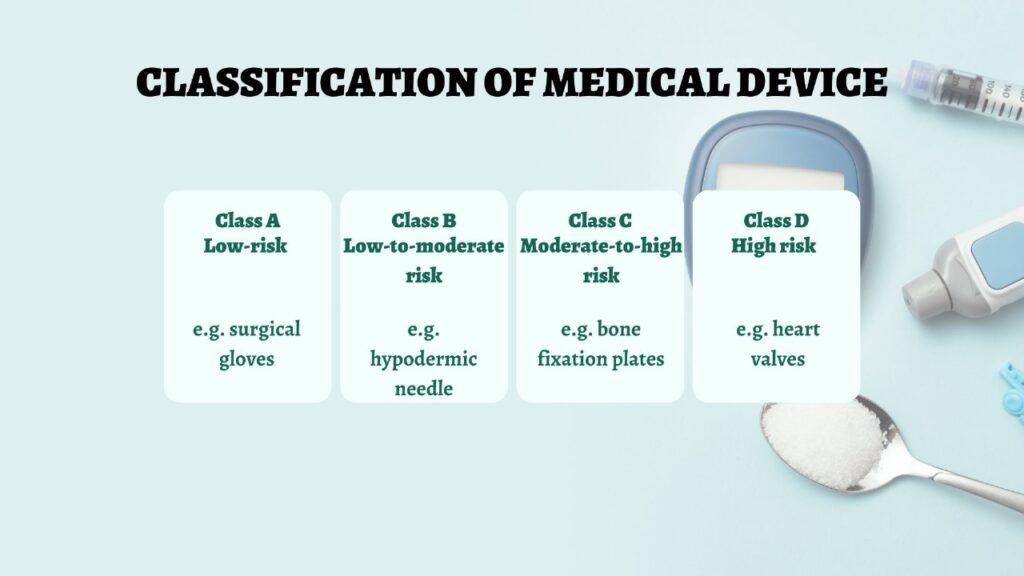
Classification of Medical Devices by CDSCO
A key initial step involves understanding the classification of your device. CDSCO categorizes devices into four classes based on risk:
- Class A: Low-risk devices (e.g., surgical gloves)
- Class B: Low-to-moderate risk devices (e.g., hypodermic needles)
- Class C: Moderate-to-high risk devices (e.g., bone fixation plates)
- Class D: High-risk devices (e.g., heart valves)
Step-by-Step Procedure for Obtaining a CDSCO Manufacturing License
- Classify Your Device: Determine the risk classification of your device (A, B, C, or D) as per CDSCO guidelines.
- Prepare Technical Documentation: Ensure the technical documents include device descriptions, manufacturing processes, specifications, safety, and performance data as required by CDSCO.
- Submit Application: Submit your application, along with technical documents, a cover letter, and fees.
- CDSCO Review: CDSCO reviews the application and may issue a deficiency letter requesting additional information if needed.
- Facility Inspection: CDSCO inspects your facility to ensure compliance with regulations.
- License Issuance: If all requirements are met, CDSCO grants the manufacturing license.
- License Renewal: Typically valid for five years, the license must be renewed before its expiration.
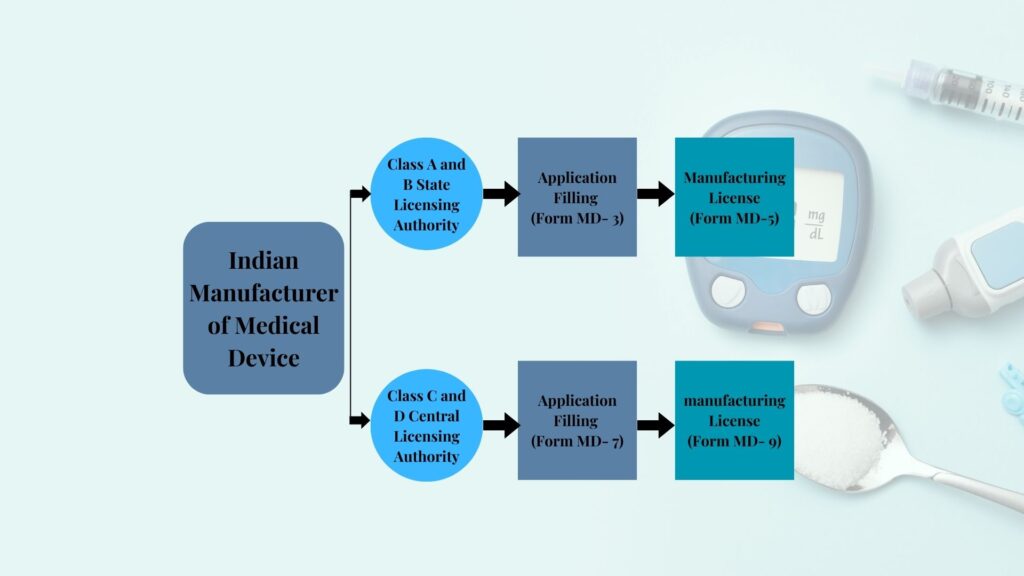
Overview of CDSCO Forms for Medical Device Registration
- Form MD-3: Application form for manufacturing Class A or Class B devices.
- Form MD-5: License issued to manufacturers of Class A or Class B devices.
- Form MD-7: Application form for manufacturing Class C or Class D devices.
- Form MD-9: License issued to manufacturers of Class C or Class D devices.
Applying for CDSCO Manufacturing License on the Portal
To apply for a manufacturing license on the CDSCO portal:
- Register on the Portal: Create an account using your email.
- Select License Type: Choose the appropriate medical device license type (e.g., Class A, B, C, or D).
- Complete Application: Fill in details about your facility, quality control procedures, and manufacturing processes.
- Upload Documents: Submit required documents, such as the manufacturing plan and product specifications.
- Pay Fees: Use the portal’s online payment system to pay the application fees.
- CDSCO Review: After submitting the application, CDSCO will review it and inspect the facility before issuing the manufacturing license.
How Bioexcel can help ?
Bioexcel Lifesciences & Research LLP can support medical device manufacturers in navigating the complex process of obtaining a CDSCO Manufacturing License through the following services:
- Regulatory Guidance and Compliance: Bioexcel provides expert advice on the classification of medical devices, ensuring that manufacturers accurately identify the license type (Class A, B, C, or D) based on the CDSCO guidelines. They also offer assistance in understanding the legal requirements, including adherence to the Medical Device Rules, 2017.
- Document Preparation and Submission: Bioexcel helps companies prepare all the necessary documents, including the Site Master File, manufacturing processes, and technical documentation required for submission to CDSCO. They ensure that the application forms (e.g., Form MD-3, MD-5, MD-7, and MD-9) are correctly completed, reducing the risk of delays or rejections.
- Facility Compliance and GMP: Bioexcel assists manufacturers in ensuring their facilities meet the Good Manufacturing Practices (GMP) standards required by CDSCO. They guide clients through the preparation for facility inspections, ensuring compliance with CDSCO’s stringent regulations.
- Personnel Training: Bioexcel offers training programs for technical staff to ensure they are qualified to manage the production processes, which is a key requirement for obtaining a CDSCO manufacturing license. This includes training on maintaining documentation, managing audits, and adhering to manufacturing standards.
- Post-Market Surveillance and License Renewal: Once the manufacturing license is obtained, Bioexcel assists in setting up post-market surveillance to monitor device performance and safety. Additionally, they help in renewing the license before its expiration, ensuring uninterrupted manufacturing.
- Regulatory Representation: Bioexcel can act as a regulatory representative, ensuring that all interactions with CDSCO are handled professionally, including responding to deficiency letters and managing facility inspections. By leveraging Bioexcel Lifesciences’ expertise, manufacturers can streamline the entire process of obtaining and maintaining a CDSCO manufacturing license, allowing them to focus on innovation and production while ensuring full regulatory compliance.
Conclusion
Obtaining a CDSCO manufacturing license is essential for medical device companies looking to operate in India. By following the outlined process and ensuring compliance with CDSCO regulations, companies can ensure smooth registration and approval for their products in the Indian market. For expert help throughout this process, contact Bioexcel, a leading medical device research organization.




